
Authors: Michael Begley and Scott Silverstein, Veolia Water Technologies
Featured in Chemical Engineering Magazine - March 1, 2022
The emergence of new products and markets is driving a global renaissance of the pulp-and-paper industry due to increased global demand for cardboard and paper packaging products, as well as the rise in the need for hygiene products, such as paper towels, tissues and nonwoven wipes. To adapt to these drivers and meet this steep demand increase, pulp producers and integrated paper manufacturers have been racing to expand their pulping operations. In addition to these increased production demands, producers are also feeling pressure from regulatory agencies, as well as consumers, to continually identify process improvements to enhance their sustainability efforts.
Pulping facilities require a great deal of water, chemicals and energy, which creates many challenges when it comes to sustainability. Although the modern pulping process includes sophisticated closed-loop processes with the ability to recover and reuse water, chemicals and energy, manufacturers should always keep a keen eye for additional opportunities that allow companies to lighten their environmental footprint. One of the key areas pulping facilities can target is to incorporate sustainability projects within the plant’s ash-treatment recovery loop.
By enhancing the ash-treatment recovery process with capital improvements, companies can generate an advantageous proposition. In addition to improving overall plant efficiency, sophisticated ash-treatment projects can significantly reduce discharged waste materials and extend the lifespan of critical utility equipment, such as plant boilers — all top-of-mind issues for mill managers. On the sustainability side, enhanced chemical recovery within the ash treatment process has the potential to yield valuable byproducts, such as high-quality fertilizers.
Though these sustainability-focused projects may appear aggressive and require investments in new technologies or process changes, in many cases, they can significantly improve overall operational performance within the pulping process and achieve a quick payback on the capital investment. As a result, both greenfield and existing pulping facilities can harvest the benefits from implementing upgrades to their chemical-recovery and ash-treatment operations.
CHEMICAL RECOVERY AND ASH TREATMENT
In most modern pulping mills, during the kraft pulping process, wood is cooked with water and chemicals at high temperatures in order to separate the cellulose from lignin and hemicelluloses. The resulting pulp is further washed with water. The water used to wash the pulp, plus the cooking liquor from the digester, is called “black liquor.”
Black liquor is concentrated using evaporation in order to be combusted as a form of renewable energy within the recovery boiler. Combustion of the black liquor’s solids leads to the generation of energy and steam, which is used elsewhere within the plant. Contained in the molten salts (smelt) are most of the needed pulping chemicals, which are collected from the recovery boiler and can be reused in the digester.
This closed circulation loop maximizes the economics of chemical recovery, reducing chemical discharge and raw chemical makeup, but also creates challenges for handling and treating liquor streams. Non-process elements (NPEs), including chloride and potassium entering the mill through the raw wood and chemical makeup, accumulate in the recovery cycle. Over time, they create conditions for scaling and plugging in the boiler, leading to lower energy production. If left uncontrolled in the precipitator ash, NPEs can become a significant problem — causing corrosion and boiler fouling — ultimately resulting in a reduction in recovery boiler capacity and an increase in operating costs.
By integrating advanced chloride- and potassium-removal systems to treat the precipitator ash, mills can unlock significant operational benefits and reduced chemical costs, while simultaneously generating additional revenue streams from produced byproducts that also increase the sustainability of the production facility.
CHLORIDE REMOVAL PROCESS
A two-stage crystallization process can be used for enhanced removal of NPEs. The first stage consists of a conventional chloride-removal process (CRP) system (Figure 1) operating close to atmospheric pressure, followed by a second-stage crystallization process designed to target potassium within the chemical recovery process.
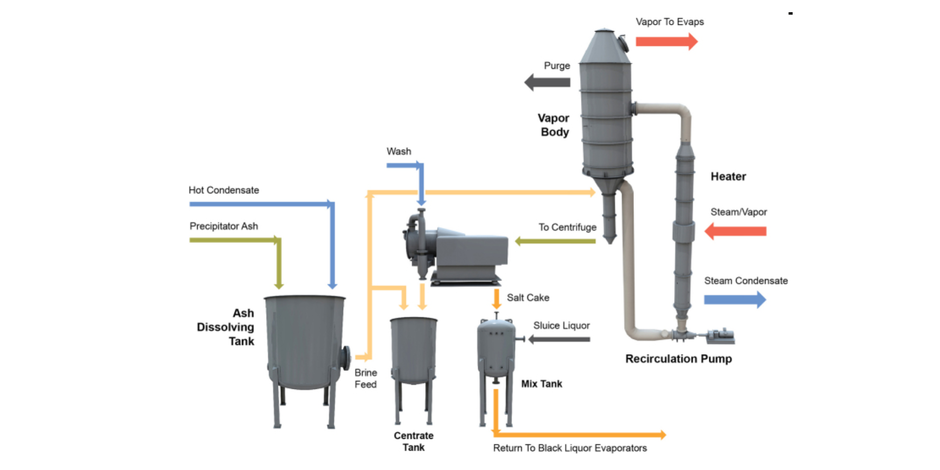
Stage 1 — CRP. Precipitator ash is dissolved with condensate, resulting in a slightly under-saturated solution feed to the CRP system. The CRP system operates as a true crystallizer, with proper crystal generation, growth and washing to maximize the removal of contaminants and recovery of sodium salts. Crystallization occurs through evaporation of water, causing the solubility limit of sodium salts to be exceeded. The resulting sodium salt crystals are dewatered and washed in a centrifuge and returned to the recovery cycle via sluicing with black liquor. Chloride and potassium remain in solution and are purged from the crystallizer in a concentrated stream with minimal sodium losses. The CRP system is typically thermally integrated with a mill’s black-liquor evaporation system in order to achieve low operational costs.
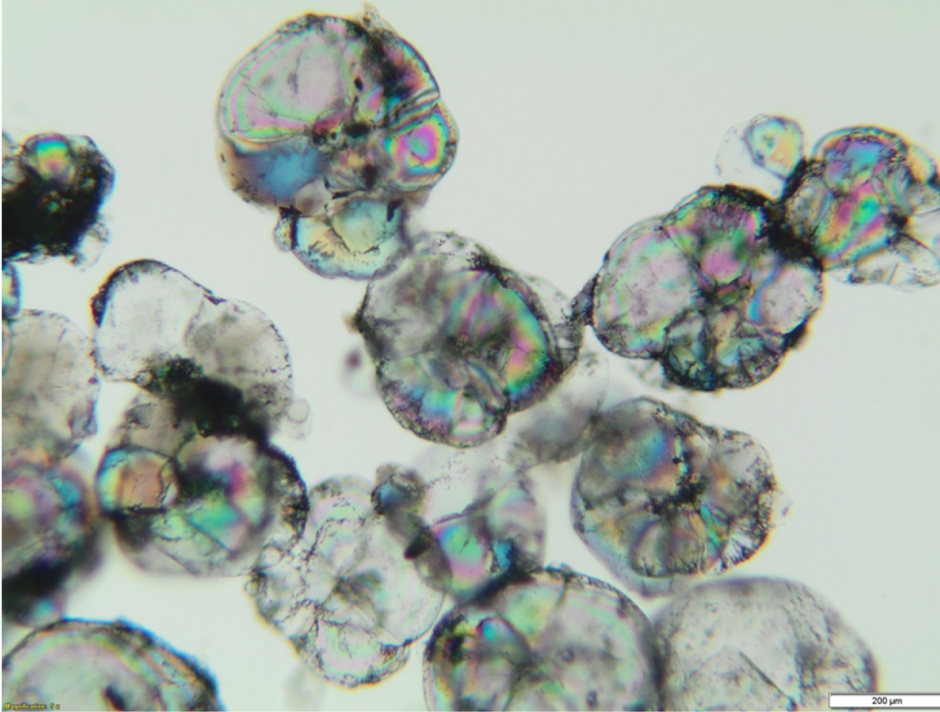
The conventional CRP system will achieve a maximum chloride-removal efficiency of 98–99% at a sodium recovery of 85–95%. For mills with higher potassium levels, the sodium recovery will be lower. For the vast majority of pulp mills, the crystallization chemistry will exceed solubility limits for potassium before reaching the chloride solubility limit. In this case, a portion of the potassium present will crystallize as glaserite (3K2SO4·Na2SO4), which is shown in Figure 2. The glaserite will be returned to the mill cycle with the other sodium salts, thereby reducing the CRP system’s overall potassium-removal efficiency. As most pulp mills are more focused on chloride reduction, the CRP system typically will be operated in a way to maximize chloride removal and soda recovery, while the potassium removal generally ends up in the 70–90% range. Pulp mills with high potassium inputs may instead wish to maximize potassium removal and can be limited to 60–80% sodium recovery when optimal potassium removal is targeted. Most high potassium mills must balance potassium removal and sodium recovery in order to minimize the cost of sodium losses.
As a true crystallizer, the conventional CRP system is a highly selective purification process to maximize chloride removal efficiency and achieve high recovery of sodium salts. Mills that have a high level of potassium in their chemical recovery loop have a unique challenge and opportunity. By implementing a second stage to the CRP, mills can produce additional byproduct streams while simultaneously removing high levels of chloride and potassium.
Stage 2 — Potassium-handling enhancements. For mills with high levels of potassium in the chemical-recovery loop, a second stage can be added to complement the initial CRP stage (Figure 3). After the CRP stage, which is operating close to atmospheric pressure, sodium salts, such as sodium sulfate, burkeite (2Na2SO4·Na2CO3) and sodium carbonate, are crystallized in the first stage and returned to the recovery cycle. The second-stage crystallizer acts as an adiabatic flash-crystallizer unit operating at lower temperature to take advantage of the reduced glaserite solubility. Crystallized glaserite solids are dewatered in a centrifuge for subsequent disposal or otherwise further processed for production of sulfate of potash (SOP; K2SO4) fertilizer. A portion of the glaserite crystallizer mother liquor is purged from the mill to further enhance overall chloride-removal efficiency, with the remainder of the mother liquor recycled to the first-stage CRP crystallizer.
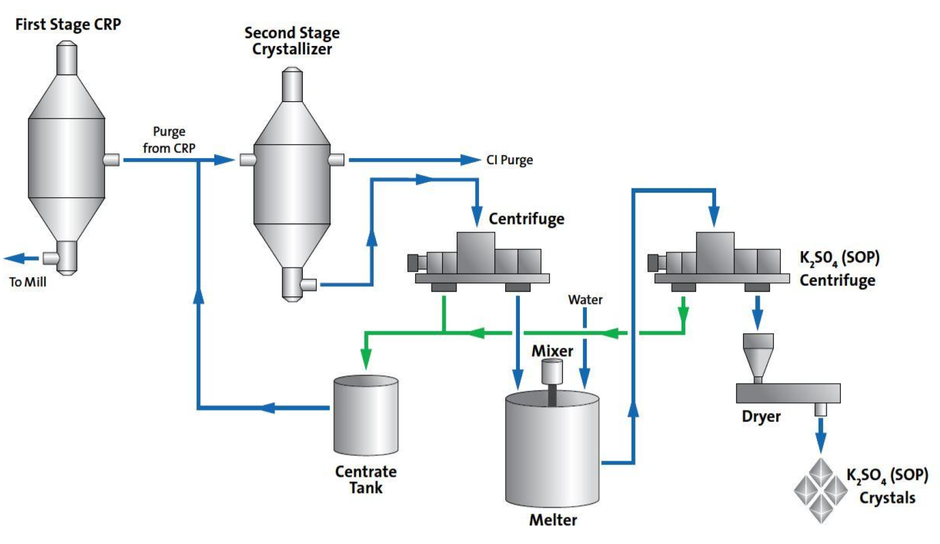
Water added to the melter results in selective dissolution of the sodium sulfate and potassium sulfate from the glaserite, leaving purified potassium sulfate in the solid phase. The potassium sulfate may then be centrifuged and dried to produce a high-quality SOP byproduct.
CASE STUDY: BRAZIL PULPING MILL
CHALLENGE
The Kraft process used in the production of pulp enables the efficient recovery of chemicals and heat in a closed-cycle process. However, it also allows the undesired buildup of chloride and potassium in the recovery cycle, which if left uncontrolled, can create corrosion and boiler fouling.
SOLUTION
After analyzing the composition and performing small-scale pilot testing, it was confirmed that the installation of an enhanced chloride removal process (ECRP) as a second stage to the site’s existing system would be able treat the additional feed of precipitator ash and prevent significant boiler-capacity losses and increased operating costs. With the new system, the site was able to treat up to 10 ton/h of precipitator ash (equivalent to 240 ton/d).
CREATING VALUE FROM BYPRODUCTS
In this particular case, the SOP produced from the ECRP system was used by the mill to fertilize its forest reserves to enhance growth rates for the next generation of trees. In doing so, the facility was able to close the loop by returning high-quality nutrient compounds back into the environment.

PRODUCING SOP
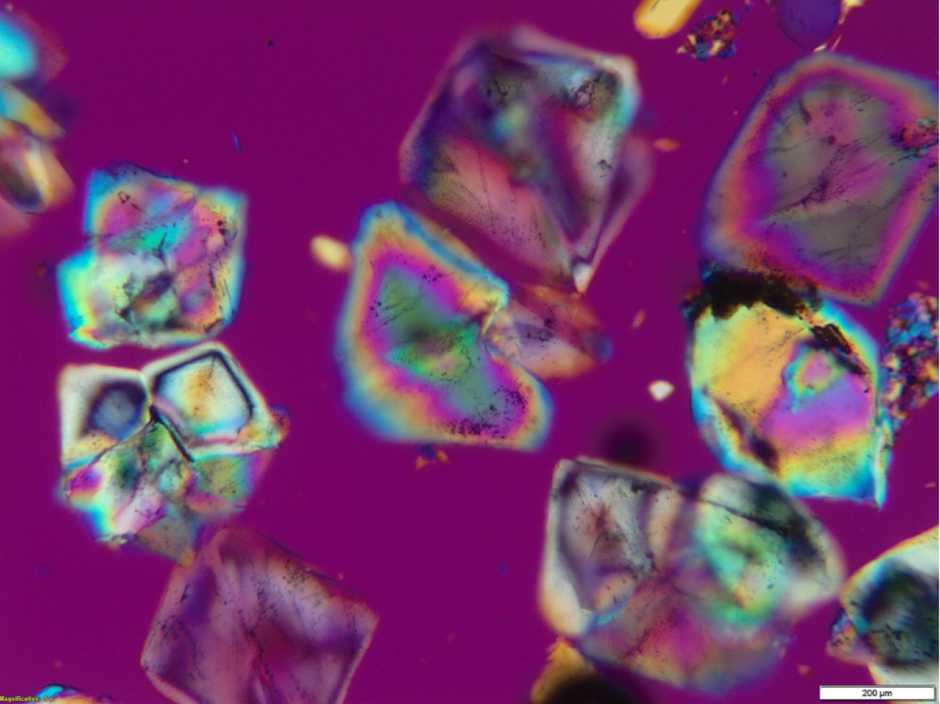
Crystallized SOP fertilizer (Figure 4) is a premium, water-soluble fertilizer that is uniquely positioned as a “smart fertilizer” that can allow for more consistent results and a higher quality crop yield. SOP’s enhanced water solubility allows the fertilizer to be injected into advanced irrigation systems, which allows for a more efficient transfer of nutrients to plants. SOP fertilizers are also gaining traction over traditional fertilizers because of their extremely low levels of impurities like sodium, chlorine and heavy metals. These impurities not only can create issues by scaling and clogging within application equipment, but they can also cause adverse effects to crop quality, especially those sensitive to chlorides.
Because of these advantages, the demand for “smart fertilizers,” such as SOP, is expected to significantly increase. This allows pulping facilities to be uniquely positioned as key contributors within the supply chain of SOP. With pulping facilities conveniently positioned across the globe, they can act as decentralized, local producers of this valuable resource. This has the potential to reduce supply-chain logistic costs and environmental impacts by shortening the distance between the source and user.
Advanced chemical recovery technologies at pulping facilities can introduce significant benefits for companies, including reduced operating costs, reduced downtime, and of course the creation of high-value byproducts, such as SOP fertilizer, which can create additional revenue streams. With respect to sustainability, these enhancement projects generally result in reduced water usage, improved energy consumption, less waste, and in the case of sites that recover nutrients and produce SOP, these sites can also play a leading role in helping secure the global food chain, as well as support fertilization of carbon-capturing forests


